Product Development:
 We partner with original equipment manufacturers (OEMs) and Tier 1 suppliers to fast-track the product development and industrialisation process. We leverage our divisional expertise in order to deliver innovative cost and time effective solutions for our partners. We are aligned with our company divisions to give a single point of contact throughout the development and industrialisation process.
We partner with original equipment manufacturers (OEMs) and Tier 1 suppliers to fast-track the product development and industrialisation process. We leverage our divisional expertise in order to deliver innovative cost and time effective solutions for our partners. We are aligned with our company divisions to give a single point of contact throughout the development and industrialisation process.
We close-loop the design process to prototype, test and validate ideas faster. We utilise our dedicated test laboratories to evaluate all work throughout the design process and where appropriate, design and validate our own bespoke test equipment. We also work with other equipment suppliers such as high volume mould and assembly machine manufacturers, contract manufacturers and secondary finishing, to expedite the industrialisation process on behalf of our customers.
-
Asthma / COPD (Chronic Obstructive Pulmonary Disease)
If you don’t have asthma, you are likely to know somebody who does – it affects over 10% of the population. Its fundamental cause is not well understood and precipitants can include pollution, pet dander, pollen, mould, tobacco smoke and chemical irritants.
- The World Health Organisation (WHO) estimates that 235 million people suffer from asthma and 64 million people suffer from COPD (2004).
- WHO also states that asthma is under diagnosed and under treated.
- The NHS (UK) spends around £1 billion a year treating and caring for people with asthma.
- COPD is the third leading cause of death in America, claiming 134,676 lives in 2010.
- An estimated 75% of UK hospital admissions are avoidable and as many as 90% of the deaths from asthma are preventable.
- Most asthma–related deaths occur in low to middle income countries.
Respiratory Drug Delivery Systems
Prior PLM Medical has been intensely involved in the development of respiratory devices for asthma and COPD. We have extensive knowledge around the challenges in delivering these products to the market and have developed a number of patented mechanisms for respiratory devices. We often engage with clients who have particular difficulties getting their products market ready. Our areas of expertise include:
- Product development.
- Independent product evaluation.
- Robust design studies.
- High speed x-ray analysis (4000+fps).
- Plume force, temperature and geometry analysis.
- High speed video analysis.
- Localised mechanism testing.
- Simulated user studies.
- Project specific test equipment.
- Device functionality studies.
- Proof of principal engineering studies.
Diabetes Mellitus
Diabetes is prevalent in the developed world and is a growing problem in developing countries. Treatment although effective can be difficult and the patient must take an active role in treating their own disease.
- Of over 220 million diabetics worldwide, 25.8 million are American.
- Diabetes is the 5th deadliest disease in U.S. claiming the lives of two thirds of all diabetics.
- 3.4 million people died as a result of high blood sugar in 2004 alone.
- WHO projects diabetes deaths to double between 2005 – 2030.
- 50% of diabetics die from cardiovascular disease, 10-20% from kidney failure.
- Between 2006-2015, it is forecasted that China alone will lose $558 billion due to heart disease, stroke and diabetes.
- 80% of diabetic deaths occur in low- to middle-income countries.
“Fusion” Project – Closed loop Insulin Delivery
Since 2011, Prior PLM Medical has been involved in an internally funded test-bed project (named “FUSION”) to develop a closed-loop insulin delivery system to help diabetic patients better manage their disease. The objective is to take the burden off the patient, empowering them by use of an implanted glucose sensor to automatically measure glucose levels (in contrast to the conventional manual “glucometer”).

- The “live” data enables a miniature discrete pump to constantly deliver insulin in a way that better replicates the normal functioning of a healthy pancreas.
- Glucose levels and insulin delivery are constantly monitored and trended. The data can be used by the patient to better understand their insulin needs and by GPs to monitor patient compliance.
- The insulin delivery pump uses an innovative peristaltic pump and disposable reservoir to deliver both small and large doses of insulin with much greater accuracy than conventional syringe pumps.
- The insulin temperature is continuously monitored to reduce the risk of insulin degradation.
- All drug contact elements are replaceable as a part of the reservoir and infusion set, maintaining sterility.
-
Design Methodology
Our product development methodology is defined by the following process:
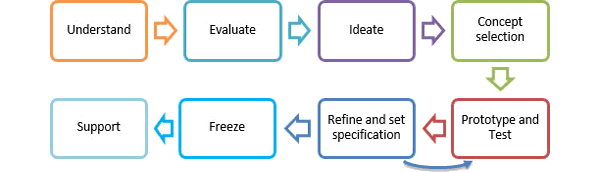
Understand
- Our process requires us to clearly define and understand our clients needs and requirements for their product and the impetus behind
the product.
Evaluate
- We conduct research in order to understand the customer needs and identify any latent needs or other market opportunities. A fact base is then used in order to help our client define a user requirement specification that will set the requirements of the device.
Ideate
- We break the project into its individual sub-components/systems/processes and address them individually. Individual and group ideation methods are used to generate ideas which are then explored systematically using classification tree and combination tree methods.
Concept Selection
- Selection criteria are carefully defined with respect to the URS with consideration to other stakeholder needs including cost, ease of manufacture, economic boundaries, market conditions etc. External and internal selection inputs including client input, user studies, target market involvement, analytical input, and research literature are examined. Concept selection is applied throughout the subsequent design and development process. Design decisions will require the URS to be further defined and the technical requirements to be developed.
Prototype & Test
- We design a test programme in order to validate the selected concepts. We use internal prototyping methods along with external partners to test physical samples as quickly as possible in order to identify and resolve issues. Using our internal capabilities, we have been able to produce prototype production quality components for device verification testing within 3 weeks of a design chill (using injection moulded cassette tool methods). Device development testing and verification testing programmes involve both comprehensive testing and localised testing using imperical and analytical methods.
Refine and set specification
- Testing can also include consumer surveys, handling studies and workshops to communicate concepts and measure customer/user response. Using in-house facilities, we can design and build bespoke validated test equipment to carry out verification testing as per designed test methods or protocols. All equipment, methods, results and ancillary information are recorded and documented as per our internal ISO audited procedures.
Support
- With our engineering knowledge, we support our customers in the industrialisation process of their product. From component renderings and animations, to supporting the specification and design qualification of manufacturing equipment, we have the expertise to streamline the process and help overcome issues as they arise. We are a dynamic and reactive organisation, committed to coming up with solutions to expedite the design process. We understand the considerable technical challenges involved in developing a medical device and bringing it to market. We can help our customers overcome these challenges and build a sustaining programme around manufacturing processes that will safeguard the quality and output of the final product.
- Our process requires us to clearly define and understand our clients needs and requirements for their product and the impetus behind
-
Test Laboratories
We have fully equipped access-controlled test labs. Capabilities and equipment include;
- Environmental temperature and humidity conditioning
- Tensile testing
- Torque testing
- High speed camera analysis
- Schlieren technique analysis
- Plume geometry analysis
- Abrasion testing
- Analytical weighing
- Component metrology and inspection.
We manage and conduct test batch assembly, root cause analysis, analytical research, localised test programmes and full device verification testing programmes.

-
Prototyping
Rapid prototypes are sufficient for basic fit, form and function but often fail to represent the quality of high volume manufactured components. Material, friction properties, surface finish, and warpage are not accurately replicated. We employ a cassette tooling system that allows us to make production quality components with a short lead-time. These samples are often used for clinical trials and to validate high volume assembly equipment in lieu of the production moulds that may still be in manufacture.
-
Digital Prototyping and Testing
Continual design validation is imperative but can be time consuming when working with third parties. Having digital prototyping and testing services in-house enables us to constantly verify our design concepts in a time efficient manner. We use in-house finite element analysis tools including Ansys and Moldflow platforms throughout the design and development process to continually validate design updates and alterations.